Business Process AutomationLearn more
Automating clinical trials with RPA - step by step guide to success
Pharmaceutical companies conducting clinical trials are dealing with multiple challenges resulting from industry specifics - highly regulated and compliance-driven. Trials are time-consuming and generate enormous amounts of data, which have to be collected, analyzed, and stored in a way assuring easy access. This article will help you to understand the potential of RPA and its benefits for streamlining clinical trials, with examples of processes that can be automated and potential benefits.
What is Robotic Process Automation and how it can be used for clinical trials?

Companies use RPA software to support employees by carrying out repetitive, rule-based tasks, which are time-consuming and error-prone. Automation is carried out through dedicated software bots that have been programmed to perform specific tasks according to rules and commands previously written in a computer program.
According to a McKinsey study, almost 45% of the jobs we do can be automated, which provides a huge potential for cost reduction and effectiveness improvement for pharmaceutical companies.
RPA software can be used to automate both front-office and back-office tasks, such as:
- filling out records and documents,
- send automatic notifications,
- make schedule entries,
- transfer data between systems,
- create documents,
- ensure data compliance.
What are the benefits of automating clinical trials?
The use of RPA software simplifies and streamlines processes that have to be conducted during clinical trials, resulting in speeding up tasks and improving results accuracy. Even regulatory agencies, such as FDA, recognized the benefits of automation and the use of new technologies, encouraging companies to implement them into their strategy, and supporting them by issuing appropriate industry guidelines.
The main benefits of using RPA for clinical trials are:
- faster completion - by automating processes each step of trials can be optimized, and study as a whole can be completed faster,
- error reduction - by automating manual tasks, that are error-prone, the risk of clerical errors can be drastically reduced,
- cost reduction - with less manual work to be done, less time to spend on them, also costs can be kept at a lower level, improving the efficiency of the whole process,
- data quality improvement - with better data comes better quality, resulting in a bigger chance of approval.
Knowing amounts that are at stake, it is easier to imagine the potential for savings that can be achieved with each improvement and automation. As estimated in research by Aylin Sertkaya, published in PubMed.gov, the cost of each phase varies, depending on the therapeutic area, spreading between $ 1.4 million for phase 1 of endocrine drugs, up to $52.9 million for phase 3 in case of pain and anesthesia drugs:

Source: PubMed.gov
Pharmaceutical companies that benefit the most from the implementation of Robotic Process Automation are the ones that start with creating an optimal strategy. It should not only focus on identifying the most promising tasks but also assure that employees are aware of the goal and potential benefits of the automation, not only for the company but also for them.
To learn more on what are elements of such strategy, what are the steps and how long does it take to implement RPA read our step-by-step guide to robotic process automation delivery.
Which areas of clinical trials can be automated?
RPA software, because of its potential to solve the problem of systems interoperability can be used in all steps of clinical trials, not only to optimize trial management and data collection, analysis, and sharing but also patient matching and processing PV cases.
The complexity and scale of clinical trials (and amount of data resulting) are well shown in the table from ResearchGate.net article on “Drugs, Devices, and the FDA”, by Gail Van Norman:
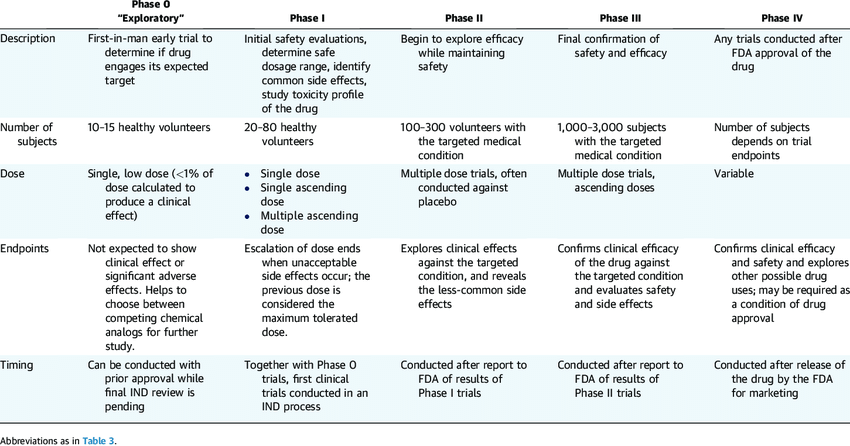
Looking in detail at all phases of clinical trials we can identify use cases in each, which can be optimized with the use of Robotic Process Automation software, including:
- regulatory affairs,
- site management related tasks (i.e. contracting, payments and monitoring),
- patient matching and retention,
- drug, biological sample and clinical supplies logistics,
- trial master file management,
- pharmacovigilance cases processing,
- human resources management, encompassing recruitment, onboarding, travel costs, salaries, and taxes.
This is by no means an exhaustive list of all areas that could be in part automated, but the complexity and repetitiveness of tasks that can be identified in them can serve as an example of the potential of RPA.
If you have specific questions about the possible implementation of RPA in your company you can benefit from a free 30 minutes consultation, during which we can help you identify your needs and pick the right technology to match your challenges.
In the rest of this article, we will go into detail about each of the areas that we identified and try to show how companies could use bots to boost productivity and reduce costs.
How can regulatory affairs in clinical trials benefit from automation?

Regulatory affairs (RA) scientists are responsible for overseeing the process of getting a drug through regulators' review and approval and onto the market. Depending on the size and scale of the company business, assuring that all regulatory requirements are being met is challenging and time-consuming.
Automation can be used to streamline regulatory management, helping RA professionals in:
- collecting and collating data from various sources and in different formats for evaluation,
- preparing submissions by gathering data from various sources and compiling them in desired formats,
- monitoring and setting timelines for approvals,
- effective preparation for regulatory inspections.
Not only monitoring or communication but also data importing and format unification can be improved with proper use of Robotic Process Automation, effecting in reducing the time needed to complete many manual tasks.
The increasing role of RPA in trial site management
Experts agree that proper site management is an essential element of clinical trial execution, improving the chance of trial success. Properly selected, prepared, and monitored sites are better in the effective recruitment, treatment, and retaining of subjects, with regard to regulatory compliance.
Many tasks related to contracting, payments, and monitoring are manual in nature and can be easily automated with RPA, not only on basic ones, such as notifications and deadline tracking, invoice collection, and even automated payments but also on a more detailed level, including:
- facilitation and review of enrollment,
- ensuring regulatory compliance and adherence to the protocol,
- management of adverse event reporting,
- review and query resolution,
- addressing data queries for database lock,
- completing final source data verification.
RPA use for patient matching and retention
According to Pharmaceutical-technology.com recruiting patients on to trials has become a bottleneck in many areas of clinical research. The procedure of creating a patient population with dynamic inclusion and exclusion is time-consuming, and can easily be optimized with the use of robotic process automation software. Bots can be used for initial patient matching, before interaction with employees will be required, in effect speeding up the recruitment process.
An illustration of automated patient-trial matching was included in article by Junyi Gao, available through Cornel University site:

Source: www.arxiv.org
With an increasing number of trials conducted with patients enrolling using the internet, the amount of data to verify grows and requires more automated solutions, especially when combined with growing interest in DTP (direct-to-patient) drug distribution strategies in trials.
DTP distribution negates patients’ needs to travel to the clinical site. While it can potentially contribute to improved patient recruitment and retention, it requires high level of automation within the supply chain of a trial drug, to mitigate risks and drive better outcomes.
Automation affects drug, biological sample, and clinical supplies logistics
Logistics involved in clinical trials are highly complex, requiring extreme flexibility and support of new technology to meet extensive regulatory requirements and remain efficient and cost-effective.
Logistical challenges which have to be overcome are only increasing, especially with new drug tracking regulations worldwide (an example of the complexity of such requirements can be the Drug Supply Chain Security Act (DSCSA) in the US).
With an increasing number of clinical trials also complexity grows:
- multiple studies are global, with sites located in numerous remote locations,
- trials are lasting longer (researching enhanced efficacy in comparison with existing products or long-term safety of treatments),
- dosing schedules are complicated,
- adaptive trial designs are used more often,
- percentage of temperature-sensitive biologic drugs in tests grows,
- DTP (direct-to-patient) strategy in trials includes not only drug delivery, but also the pick-up of laboratory samples (when blood samples are drawn and prepared for shipment at patients' homes).
A good example of a company embracing automation can be Marken, a subsidiary of UPS, shipping drugs and biological materials at all temperature ranges to investigator sites, depots, and directly to patients’ homes.
The company uses advanced tracking technology with GPS for real-time tracking of a package’s location and monitoring of any exposure to temperature variations, vibration, light, and shock - with automated notifications in case of breaching border conditions (for example by alerting airlines to move packages that were not maintained at the correct temperature).
Trial master file management with RPA software

All activities related to clinical trials have to be registered and stored in TMF, and in many cases, those data are entered manually. With proper use of RPA, all of that information can be uploaded automatically, and with proper data structure, bots can be also used to verify which data are missing.
The list of information that should be gathered in TMF is extensive, including:
- trial documents,
- details of the laboratories,
- shipment records,
- storage records,
- monitoring visit reports
- and much more.
By combining RPA software (for example UiPath) with optical character recognition (OCR), which allows converting print or handwritten text into machine-encoded text, most of the data in those documents can be easily digitized and effectively used.
Automatisation in processing pharmacovigilance cases
According to an Ernst & Young report, a large pharma company processes approximately 700,000 adverse events (AE) cases annually.
It’s worth noting that:
- 50% of PV resources are currently spent on managing cases that require integration of data that vary in quality, structure, and format,
- by Ernst & Young estimations by automating such manual steps the typical top biopharma company can reduce time spent on PV by 45%, with potential multimillion-dollar annual savings.
By implementing RPA software data collection and management can be radically improved, increasing effectiveness and guaranteeing significant cost reduction.
Other common applications of RPA software in companies conducting clinical trials
As mentioned, Robotic Process Automation has enormous potential to be applied to multiple processes carried out by any company, from simple tasks (generating an automatic response) to more complex activities. Such flexibility allows this software to be implemented in basically any department within the company, where repetitive tasks are performed.
Most frequently described use cases of RPA include:
Administration, tax, and finance department:
- accounts payable,
- payment processing,
- bank reconciliation,
- tax reporting.
Human resources department:
- travel and expense management,
- employees master data management,
- time record validation.
Financial and accounting operations are especially good candidates for automation, as they are largely rule-based, very manual, and virtually always carried out within IT systems. This is confirmed by the 2019 McKinsey report, pointing out various business areas and their potential for business process automation:

RPA as a part of a strategy to automate processes and workflows
In this article, we focused on a small part of the automation strategy, which is the use of RPA software for streamlining manual and error-prone processes, which can be identified and linked to all phases of clinical trials.
It’s worth noting that it would be impossible to find today a pharmaceutical company that did not implement any sort of electronic document workflow. But with all the changes that are happening within the industry, it is essential to ensure that such document circulation systems still properly serve their purposes, which are most of all:
- eliminating human error from the document flow process,
- ensuring control over the document circulation processes,
- creating a central repository for documents,
- putting in order messy and unstructured processes.
Many companies have implemented an electronic document workflow without proper thought. That is why we can find even today companies that struggle with outlandish and intricate processes executed in an even more outlandish way.
To learn more about what a company should consider to assure that automation is being implemented and used in the most effective way, we invite you to read our in-depth guide on How to streamline and automate processes and workflows in your company.
How can we help your enterprise in Robotic Process Automation?

With our experience from working on complex projects, as RPA consultants we are able to guide a company through the entire process of RPA implementation, from creating a strategy, discovering processes, designing optimal configuration, testing, up to full-scale use, and effectiveness monitoring.
GSS IT Consulting experts can support enterprises in starting digital transformation with:
- defining strategy,
- analyzing, understanding, and documenting business processes,
- setting up operating models,
- identifying technical infrastructure requirements to deploy automation solutions,
- educating employees and helping to create an “RPA-friendly” environment, where each team member looks for more automation opportunities to support company transformation,
- creating and documenting test scenarios and procedures to ensure optimal configuration,
- training team members in the building, operating, and maintaining automation solutions,
- helping to solve issues that arise in day-to-day operations with RPA software,
- further support of the implementation of any type of automation technologies by the company,
- helping to plan and set up Centers of Excellence or Process Mining Hubs, to boost up automation efforts.
If you wonder how to start or improve the implementation of automation and digitization of processes and document flow in your company, contact us for your 30 minutes free consultation.
After completing the form, we will arrange a call at a time convenient for you. During the call, our consultant will suggest how to approach the Robotic Process Automation initiative in your Company. Thanks to this, you will assess which technologies will bring you the greatest benefits in the area of automation and standardization of processes.
